Today Lex Machina announced the release its first annual Products Liability Litigation report which analyses more![]() than 400,000 product liability cases filed in U.S. District Courts from January 1, 2009 to Dec 31, 2017.
than 400,000 product liability cases filed in U.S. District Courts from January 1, 2009 to Dec 31, 2017.
The report highlights claims related to pharmaceuticals and medical devices and provides detailed insights on case findings, resolutions timing, damages awarded in verdicts, and approved class action settlements.
Owen Byrd, General Counsel and Chief Evangelist is quoted in the press release announcing the report. “Every year, the number of product liability cases filed in District Court consistently outpaces the combined case filings for patent, commercial, employment, trademark, copyright, antitrust, securities, and bankruptcy appeals. Of those product liability cases, medical device and pharmaceutical litigation accounts for the lion’s share of all filings, which is why we’ve chosen to feature this sector in our first annual report.”
Key Findings Include:
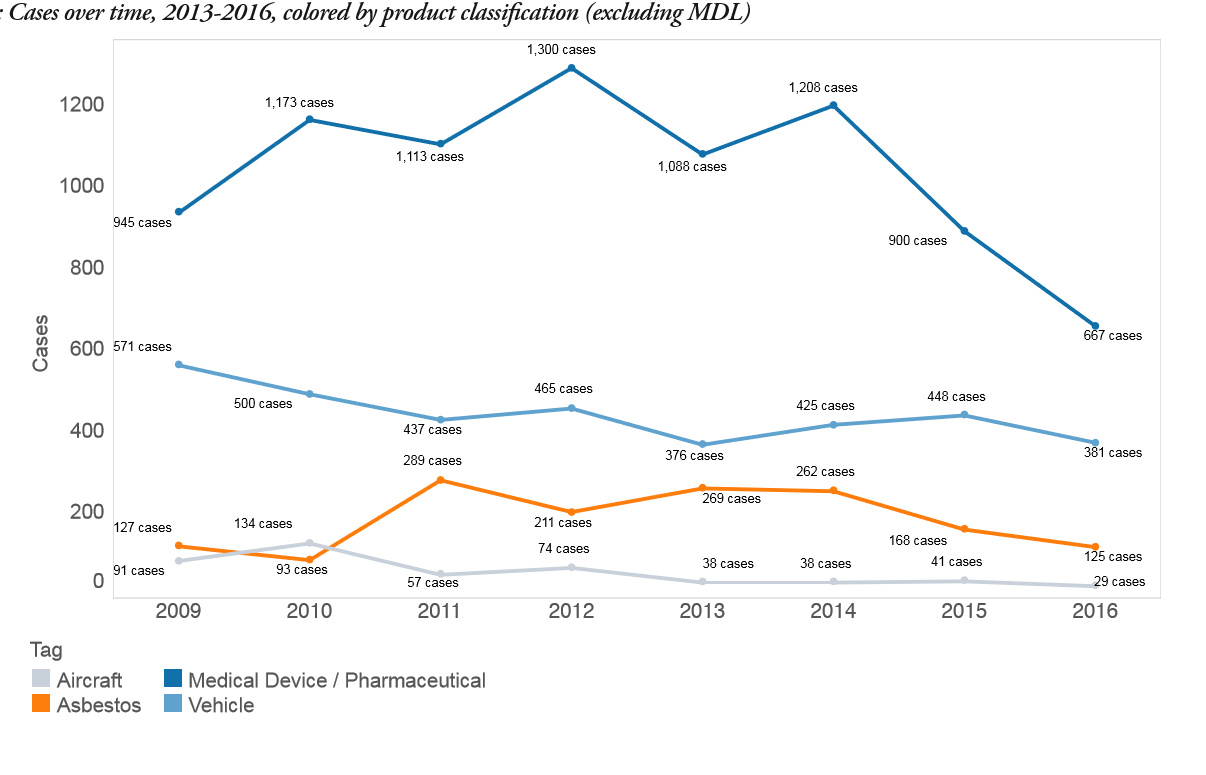
- MDL-associated cases comprised more than 97% of medical device/pharmaceutical cases filed in 2017, with only 667 of the 29,185 total cases not affiliated with an MDL master case.
- Since January 1, 2009, more than 289,200 product liability cases were filed in District Court, involving products classified by Lex Machina as medical devices or pharmaceuticals, including MDL-associated cases. The next largest category was asbestos, with more than 87,300 cases. Asbestos case filings in District Court have declined from tens of thousands per year during 2009-2011 to merely hundreds per year during 2012-2016.
- Almost all (96%) product liability cases involving medical devices or pharmaceutical products are settled or resolved procedurally, instead of on the merits of the case. When product liability cases are decided on the merits, defendants prevail about 90% of the time.
- Cases litigated through to court award of damages are rare in product liability cases involving medical devices and pharmaceutical products.
- Three districts tied for the largest percent of non-MDL filings across all product categories from 2009 to 2017: Central District of California (C.D. Cal), Eastern District of Pennsylvania and District of New Jersey – each with 5%.
- Since 2009, New Jersey has had the most non-MDL-associated medical device/pharmaceutical case filings (8%). C.D. Cal and Eastern District of Louisiana are tied at 6%, and Northern District of Texas captured 5%. This data includes cases that may later become associated with an MDL.
Expert Witness Explorer
Lex Machina also announced a new Litigation Analytics “app.” The Expert Witness Explorer is the eighth specialized “app” and provides template driven access to data and insights about the use of expert witnesses or reports. Sample analytics include determining the number of times an expert or report has been admitted, limited, or excluded from a case; the most common experts and the parties that retained specific experts, how judge rulings have favored plaintiffs or defendants.
Analytics : Where its all About the Questions
Either I paid more attention to the 5 pages of methodology or Lex Machina has enhanced the methodology section of their statistical reports. The “methodology” pages provide an outline of issues that should be raised with any analytics provider. I don’t recall seeing this level of detail in LM this earlier surveys. As analytics become a more important tool of legal analysis, attorneys and researchers must understand the parameters of each analytics system on the market. A read through the methodology section provides a good overview of the variety of issues that are impacting the validity or limitations of the analytics in the product.
While Lex Machina does use the nature of suit codes from the Pacer system, the report lists the eight Pacer NOS codes that they include in their definition of a products liability case. Lex Machina also analyzes Pacer complaints to capture cases filed without an NOS code or coded improperly. This allows them to eliminate cases that do not involve products liability despite being coded that way. The additional ways they supplement the data from Pacer includes correcting errors such as spelling mistakes and normalizing data on judges, parties, law firms and attorneys. They extract the records of law firms and attorneys not found in the basic data. They tag, categorize and annotate case resolutions with damages.
The methodology section includes a glossary – effectively educating users on how Lex Machina defines the most important aspects of products liability cases including concepts such as injury, product and defect. They define the subcategories and case tags which are included in the analysis. The report defines the 16 specific products liability findings and five damages types which are analyzed.
As we all enter the brave new world of analytics – the understanding of data parameters and algorithms will be of increasing importance.This analysis must become second nature to all lawyers librarians and researchers.